© Kirk Feral 2024, All Rights Reserved. These materials may be duplicated and shared for educational purposes only. No part of this website may be duplicated or distributed for profit, for commercial purposes, or for posting to another website, without the expressed written consent of the copyright holder.
Magnetism in Gemstones
An Effective Tool and Method for Gem Identification
© Kirk Feral
Ferroaxinite
The chemical formula for Ferroaxinite - Ca2Fe2+Al2BSi4O15OH – specifies that iron (Fe2+) is the defining transition metal. Ferroaxinite was the first species of Axinite to be discovered. When the new mineral name Axinite was first adopted in 1797, other species in the mineral group were not known. The species name Ferroaxinite was not declared until 1909. Ferroaxinite is an idiochromatic (self-colored) species, colored primarily by bivalent iron (Fe2+) that produces brown color.
Ferroaxinte & Manganaxinite
Color can be modified somewhat by the presence of additional coloring agents such as manganese (Mn2+ and Mn3+) and vanadium (V3+), resulting in yellowish brown, pinkish brown, and purplish brown body colors. Using a dichroscope, two or three colors (pleochroism) can often be seen in Ferroaxinite, and pleochroic colors can include yellow, pink, brownish purple, and sometimes green.
Pinkish Brown Ferroaxinite 3.2ct, Pakistan
SI 599, Drag
The 1.05ct brown Ferroaxinite cushion shown below has dark brown color and high magnetic susceptibility (SI 618). Although Ferroaxinite is not considered a color change species, we find that a noticeable color change under incandescent light toward brownish pink, brownish red or brownish orange is apparent in most Ferroaxinite gems and crystals. The pink/orange color in incandescent light may be related to the presence of trivalent manganese (Mn3+), which can be present in Ferroaxinite (Chalmin, E. et al., 2008).
Ferroaxinite in Daylight & Incandescent Light
1.05ct, SI 618, Drag
Color zoning is the unequal distribution of color, either body color or a secondary color. Secondary colors do occur in the Ferroaxinite species, although they are uncommon and can be obscured by dark brown color. Unlike pleochroism, where different colors are visible when the gem is viewed at different angles, color zoning occurs as discrete patches or streaks of color within the body of the gem. Below is an example of blue color zoning in dark brown Ferroaxinite, as it appears in transmitted light. The blue color is the result of vanadium irregularly distributed at the end of a 2 mm thin Ferroaxinite crystal from Pakistan.
Blue Color Zoning in Ferroaxinite
Because iron quenches UV fluorescence, all Ferroaxinites are inert to UV light. Since iron oxide (FeO) is highly paramagnetic, Ferroaxinite gems typically show a Drag response to an N52 neodymium magnet and have the highest magnetic susceptibilities among the three gem Axinite species. Based on our magnetic susceptibility measurements, we estimate the potential range of susceptibility for the species to be SI 325-650, with measurements averaging around SI 600.
Ferroaxinite can blend with Magnesioaxinite, and as magnesium replaces iron (Fe2+), the magnetic susceptibility decreases, and color intensity can become lighter. Intensity of brown color therefore may be at least somewhat related to the concentration of Fe2+.
The crystal shown below left has the lightest color of any brown Axinite in our study and correspondingly has the lowest measured susceptibility of only SI 360. This appears to be an example of Ferroaxinite with lower iron (Fe2+) content replaced by a significant amount of magnesium from Magnesioaxinite. The crystal is part of a parcel of 28 light brown Axinite roughs from Pakistan, all with similar low magnetic susceptibility.
We were also able to test an anomalous example of a Ferroaxinite from Russia with black body color. In daylight, the faceted gem shown below appears black and opaque. Under magnification the gem is translucent at the facet edges. The black body color and opacity are due to a dense suspension of fine particle inclusions. This unusual Axinite gem shows color change from black in daylight to olive green in cool LED light.
Black Axinite in Daylight (lft) & Cool LED Light (rt), 2.13ct, Russia
SI 999, Pickup
Manganaxinite
Manganaxinite - Ca2Mn2+Al2BSi4O15(OH) – is a manganese-rich form of Axinite that was first recognized in 1891 and officially named in 1909. Manganaxinite is an idiochromatic species that mixes freely with Ferroaxinite in a continuous solid solution series. Magnetic responses for Manganaxinite are the same as Ferroaxinite, with gems of both species showing a Drag response when of average weight.
Based on multiple measurements, we estimate the full range of magnetic susceptibility for Manganaxinite is SI 300-550, with most samples testing near the pure endmember at around SI 550, which is 15% lower than our estimate for the Ferroaxinite endmember (SI 650). Some brown Manganaxinites have a significant amount of iron (Fe+) from blending with Ferroaxinite, and such Manganaxinites can be indistinguishable in appearance from Ferroaxinites.
As an example, the Axinite gem pictured below has a mixture of brown and yellow color. Its refractive index RI 1.67-1.68 is typical for both Ferroaxinite and Manganaxinite. Its magnetic susceptibility (SI 545) is low for Ferroaxinite but typical for Manganaxinite. Since the color intensity is strong and undiluted, we don’t ascribe the lower SI to mixing with Magnesioaxinite. The absorption spectrum for this gem also lacks the peak at 580nm typical of Ferroaxinite. We conclude this gem is composed primarily of Manganaxinite (yellow color) mixed with Ferroaxinite (brown color).
Yellowish Brown Manganaxinite, .55ct, Pakistan
SI 545, Drag
Like Ferroaxinite, Manganaxinite is not regarded as a color change species, but limited color change from yellowish brown color in daylight to brownish orange in incandescent light can be seen in some Manganaxinites. One unusual orangey brown sample from Brazil (pictured below) showed pronounced color change to bright orange in incandescent light. The orange color is presumably due to manganese.
Unusual Color Change in Manganaxinite, 1.4ct, Brazil
SI 562, Drag
Golden yellow and brownish yellow body color in Manganaxinite gems is uncommon, and color is no doubt the result of a combination of Mn2+ (yellow) along with a small amount of Fe2+ (brown). These light-colored yellow gems show no color change from daylight to incandescent light, and they also fail to fluoresce due to the presence of iron. All the yellow Axinite gems we tested were light in color and had significantly lower magnetic susceptibility (near SI 350) than other Manganaxinites, indicating lower manganese content and higher magnesium content from Magnesioaxinite. We did not find any yellow Manganaxinites that were near the Manganaxinite endmember in composition.
In Manganaxinites that approach pure endmember composition, iron (Fe2+) is mostly absent, and we find examples where brown and yellow color are completely absent. Other colors produced by trace amounts of transition metals then become more discernable or dominant in Manganaxinite. A depletion of iron (Fe2+) also permits UV fluorescence, a distinguishing feature of Manganaxinites that approach pure endmember composition.
Some Manganaxinite gems are pale in color because bivalent manganese (Mn2+) is a weak coloring agent and may produce little or no color even when the concentration of Mn2+ is high (up to 10% MnO by weight). We find an analogous situation in Pyrope-Spessartine Garnet.
Another weak coloring agent that may fail to produce color in Axinite is trivalent iron (Fe3+). We know this is the case in Grossular and Andradite Garnet. In Axinite, chemical research has shown that as the concentration of Mn2+ increases, so does the ratio of trivalent iron (Fe3+) to bivalent iron (Fe2+) (Andreozzi, G., 2000). The small amount of iron that remains in Manganaxinites near the pure endmember is mostly trivalent iron (Fe3+). Hence most or all the color in endmember Manganaxinites can be the result of transition metals other than Mn2+, Fe2+ or Fe3+.
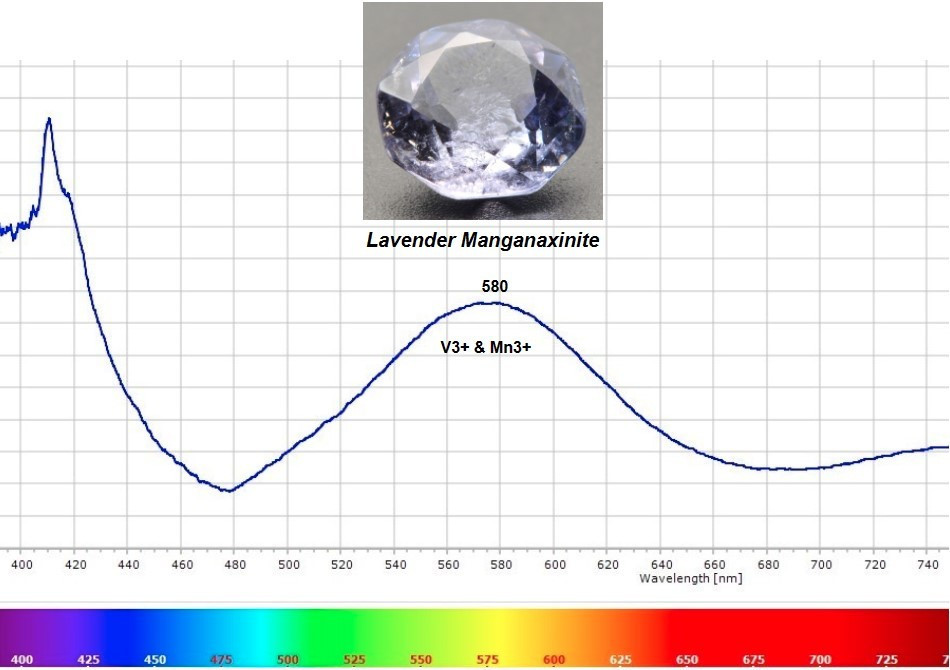
In stark contrast to bivalent manganese (Mn2+), trivalent manganese (Mn3+) is a very strong chromophore. Just a trace amount of trivalent manganese (Mn3+) can create pink color (Vigier & Fritsch, 2020), and extremely low concentrations of vanadium (V3+) can produce blue color in Axinite (Arlabosse, J. et al., 2008), A combination of vanadium and Mn3+ in trace amounts likely causes the pale lavender color seen in Manganaxinite gems such as the octagon pictured below. Fine examples of lavender Axinite gems like this are very rare in today’s marketplace and nearly impossible to find.
Lavender Manganaxinite 2.9ct, Tanzania
Daylight, SI 525, Drag
Because the gem above has no brown color (i.e. no Fe2+) and at the same time has a high magnetic susceptibility (SI 525), we can infer that magnetism is due almost entirely to Mn2+, and that chemical composition may approach the pure endmember for Manganaxinite. This gem also shows definitive color change from lavender in daylight to orangey-pink in incandescent light, and appears pink under a Chelsea filter, presumably due to the presence of trivalent manganese (Mn3+) and/or possibly chromium (Cr3+).
Lavender Manganaxinite Shows
Color Change in Incandescent Light
Lavender and pink Axinite gems show optical absorption spectra features that are different from brown and yellow Axinites whose color is controlled by iron (Fe2+) and manganese (Mn2+). The absorption spectrum illustrated below shows green and yellow light is absorbed at the broad peak in the center of the visible spectrum near 580nm. This light absorption can be attributed to a combination of vanadium (V3+) and trivalent manganese (Mn3+). Light transmission in the blue region occurs at the “valley” or transmission window on the left near 480nm, and red/pink color transmission takes place at the transmission window on the right near 680nm. Pale blue + pale pink = pale lavender.
Visible Absorption Spectrum for Lavender Manganaxinite
When longwave UV light is applied to this lavender octagon Manganaxinite gem, strong reddish orange fluorescence occurs, as pictured below. Orange fluorescence also occurs under shortwave UV light, but it is much weaker. The color of fluorescence in Axinite is believed to be the result of orange luminescence activated by manganese (Mn2+) in combination with red luminescence activated by traces of chromium (Cr3+) (Vigier & Fritsch, 2020). Similar pinkish orange fluorescence from manganese (Mn2+) and chromium (Cr3+) occurs in light-colored Grossular Garnets such as pale orange, pale green and colorless Grossular Garnets.
Lavender Manganaxinite Fluoresces Orange
Fluorescence in any type of gem or mineral is possible only when the concentration of iron is very low. The concentration of vanadium must also be very low, as vanadium can quench fluorescence as effectively as iron (Feral, K., 2019). While iron (Fe2+) and vanadium (V3+) both inhibit fluorescence when concentrations exceed trace amounts, manganese (Mn2+) activates fluorescence in Axinite.
While collecting our Axinite samples, we found frequent misidentification of light-colored Axinites such as the pale lavender gem shown above. Pale lavender Axinites from Tanzania are generally assumed to be Magnesioaxinites. Of the 9 lavender Axinites from Tanzania that we tested, all had been labelled as Magnesioaxinite, but 6 turned out to be Manganaxinite instead, showing a Drag response to our magnetic wand. Two of those lavender Axinites were from Bill Vance's collection and were mispresented in a 2017 issue of Gems and Gemology as Magnesioaxinite (Pay, D., 2017).
Lavender, lilac, or pale blue colors are indeed found in some Magnesioaxinites (Jang-Green, H. et al., 2007), but testing is of course necessary to verify the correct species. As we have shown, magnetic testing provides a simple way to separate light-colored Magnesioaxinte gems from Manganaxinite. If the Axinite is of average size and fails to show a direct response to a magnetic wand without floatation, the gem is Magnesioaxinite.
Pictured below is an unusual bicolor Manganaxinite crystal with natural internal fracture lines along the center. Pale brown color is apparent on the left side of the fracture and lavender color on the right. Although the brown color indicates the presence of iron (Fe2+), and pure lavender color signals the depletion of iron (Fe2+), the measured magnetic susceptibility on both sides of this large crystal is identical. Orange UV fluorescence on both sides is also equally strong, suggesting that the concentration of Fe2+ on the left side is too low (< 0.1 wt% FeO) to inhibit fluorescence or affect magnetic susceptibility even though it is sufficient to create very pale brown color.
Bicolor Manganaxinite, 18ct, 18 mm Wide, Tanzania, Strong UV Fluorescence Throughout, SI 556, Too Heavy to Drag
In the mines of Tanzania, lavender Manganaxinite can be found along with Tanzanite (purple Zoisite), and such Axinite crystals have been mistaken for pale Tanzanite. Both minerals are pleochroic, and the refractive index of Tanzanite (RI 1.69-1.70) is only a bit higher than that of Manganaxinite (RI 1.67-1.68). But it’s a simple matter to distinguish between these two minerals with a magnetic wand.
The small Manganaxinite gem shown below left shows a Pickup response due to its light weight (0.18ct). The Tanzanite shown below right is magnetically Inert (Diamagnetic), as we would expect of any transparent Zoisite. Another easy way to separate these look-alike gems is to check for longwave UV fluorescence. Lavender Manganaxinite shows strong orange fluorescence, and Tanzanite is inert to UV light.
Light Brown Ferroaxinite, 3.45ct, Pakistan
SI 360, Drag
Parcel of Light Brown Ferroaxinite
Drag
Yellow Manganaxinite 1.24ct, France
SI 347, Drag
Yellow Manganaxinite 4.65ct, France
SI 352, Drag
Lavender Manganaxinite .18ct Tanzania
Pink color, with no blue color component from vanadium, occurs in Axinite, although pink color has only been reported in the Magnesioaxinte species. Pictured below, we present what we believe is the first reported and documented example of pink Manganaxinite. This pale pink crystal appears identical in color to pale pink Magnesioaxinites that we have tested, but the high magnetic susceptibility (SI 477) of this crystal clearly distinguishes it as Manganaxinite. The crystal also shows strong LWUV orange fluorescence and has a specific gravity of 3.22.
Pink Manganaxinite, 5.27ct, 16 mm Tall
SI 477, Drag
Color zoning can be found in all 3 species of gem Axinite, but perhaps the most striking examples are seen in Manganaxinite gems. Since Manganaxinite can be light in color, instances of color zoning stand out more clearly than in Ferroaxinite. The rarest colors found in Axinite are blue and green. Blue color zoning is due to traces of vanadium that are unevenly incorporated into the crystal. The Manganaxinite gem shown below has areas of dark blue color within a transparent light gray body.
Blue Zoning in Manganaxinite 1.05ct, Pakistan
SI 543, Drag
Green color may be the rarest color found in Axinite. Although the cause of green color has not been specified or documented in published literature, we suspect a trace amount of chromium (Cr3+) might be the source of color, as chromium frequently produces green color in gems and is known to be a trace element in Axinite. The light-colored brownish-yellow Manganaxinite gem pictured below has green color zoning. This gem was incorrectly sold as Magnesioaxinite, presumably because of its light body color.
Green Zoning in Manganaxinite .6 ct, Pakistan
SI 545, Drag
The remarkable oval Axinite gem pictured below contains zones of blue and green color cohabiting the same stone. This gem has a near-colorless yellow-gray body and was also incorrectly sold as Magnesioaxinite, but a Drag magnetic response immediately rules out Magnesioaxinite. The light body color in conjunction with high magnetic susceptibility (SI 564) informs us that this gem is Manganaxinite with no significant Magnesioaxinite content.
Blue and Green Zoning in Manganaxinite, 2.16ct, Pakistan
SI 564, Drag
Manganaxinite gems with solid blue, solid green or solid orange body color were not found during the course of our study, but the existence of such gems is certainly possible.
Black Axinite from the same source as the gem above have been analyzed by Raman spectroscopy, and results were published in the Journal of Gemmolgy (Laurs, B., 2016). The inclusions were identified as Clinochlore, which is a greenish mineral that can have high iron content due to mixing with idiochromatic Chamosite. Our magnetic measurements are consistent with unusually high iron content. The magnetic susceptibility of the black Axinite in our study is remarkably high at SI 998, while typical brown Ferroaxinite is near SI 600. It should be noted that faceted gemstones of any kind that contain magnetic inclusions that affect magnetic response or magnetic susceptibility measurements are rare.
Lavender Tanzanite .87ct Tanzania