Black, Orange, Blue, Green
Black: In mineral form, black is the most common color of Tourmaline found in nature. Black Tourmalines are seldom faceted as gemstones today, although they were common in mourning jewelry in the past. Most black Tourmalines belong to the Schorl species, which is opaque and idiochromatic, with a high concentration of iron. Schorl owes its color and lack of transparency to high concentrations of 3 transition metals: 1) iron within its chemical formula, 2) manganese impurities, and 3) titanium impurities. Titanium contributes color through inter-valence charge transfer (Fe2+-Ti4+). Charge transfer processes involving other ions also contribute to the black color. Black Tourmalines show a Drag response. Quantitative measurements of magnetic susceptibility tell us that opaque black Tourmalines are by far the most magnetic of all the color varieties of gem Tourmaline.
Black Tourmalines
(2.42ct. & 2.23ct., Drag, SI 990 &1150)
Tourmaline species are classified according to chemical compositions that are often unrelated to color or magnetism. Different species generally can’t be distinguished from one another by their color or magnetic responses or magnetic susceptibility measurements. Gems of different species can have the same color. The transition metal impurities and charge transfer processes that appear in one species may also appear in other species. For example, not all black Tourmalines are Schorl. Some Dravite and Uvite gems are also black. Another example is brown and orange Tourmaline, as discussed below:
Brown and Orange: Dravite is typically a brown or orangey brown Tourmaline colored by iron-to-titanium charge transfer (Fe2+-Ti4+). The iron is derived from mixing with Schorl. Iron ions in brown Tourmalines are often not magnetically detectable because only very low concentrations of iron are needed to create color through the iron-titanium (Fe2+-Ti4+) charge transfer process.
The 3 brown Tourmalines pictured below show an Inert (Diamagnetic) response to an N52 magnet. Their color and lack of magnetic attraction are not specific to the Dravite species alone. Elbaite, Liddicoatite and Uvite gems can also be brown, presumably due to the same iron-titanium charge transfer process. Some of the brown Tourmalines in our study have been identified by Raman spectroscopy as Elbaite. However, for the sake of simplicity we will assume that most of the brown Tourmalines shown below are Dravite.
These Brown Tourmalines are Diamagnetic
Identifying Chrome Tourmaline: Dark green Chrome Tourmalines and Verdelite Tourmalines colored by iron can sometimes look alike (see comparison below left). Chrome Tourmalines with light green colors due to lower concentrations of vanadium and chromium can look identical to pale green Tourmalines colored by iron (see the oval and rectangle gems below right). Fortunately, we can use magnetic response to easily separate these 2 Tourmaline species whether they are light green or dark green.
Chrome Tourmalines (Dravite species) of any color intensity usually show an Inert (Diamagnetic) response, or in some cases very weak attraction. Only 2 of 11 Chrome Tourmalines tested in our study showed slight magnetic attraction. In contrast, green Elbaite Tourmalines of any color intensity caused by iron (Fe2+) show significant magnetic attraction, either via the Direct Method or Floatation Method.
Chrome Tourmaline (.86ct., Inert, SI <0) & "Verdelite" (.79ct., Drag, SI 317)
Blue: Most blue Tourmalines (Elbaite species) are colored by ferrous iron (Fe2+) as well as iron-to-iron charge transfer (Fe2+ - Fe3+). The iron is derived from mixing with Schorl in solid solution series. On rare occasions, Tourmalines are formed in a geologic environment that is high in copper. The copper will contribute blue color in such Tourmalines, which have been given the trade name "Paraiba" Tourmalines (discussed on page 4).
Magnetic response can identify blue "Indicolite" Tourmaline, which is the trade name for Tourmaline with indigo blue, pure blue or blue-green color. Most Indicolites belong to the Elbaite species, but Raman spectroscopy identifies the oval gem below (right) as Liddicoatite. "Indicolite" is colored by iron, and a Drag response is diagnostic for this variety. "Indicolite" is the only blue gemstone of any kind, not just Tourmaline, that will show a Drag response.
Often blue and green colors mix within a single Tourmaline gem. Strictly speaking, only pure blue or pure indigo gems such as the pear and oval gems above are "Indicolite", but pure blue gems are seldom seen. Most dark blue gems referred to as "Indicolite" contain some amount of green, like the blue-green cushion shown above (center).
The square gem pictured in the photo below (left) seems to have equal amounts of blue and green color. It shows a Drag response, but should we refer to it as "Indicolite"? The bi-color oval in the photo below right is predominantly dark blue, but it also has some green and yellow color. It shows only a Moderate response, indicating much lower iron content than what we see in "Indicolite". These are just two of many examples of Tourmalines in shades and mixtures of colors that do not neatly fit any single color variety or trade name.
Greenish Blue Tourmaline
(2.59ct., Drag, SI 304)
Blue & Green Tourmaline
(2.85ct., Moderate, SI 91)
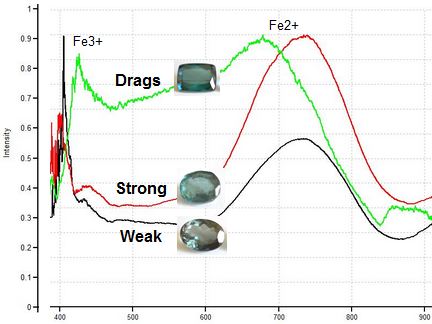
Our study of Tourmaline magnetism uncovered other surprising results. As an example, not all dark blue Tourmalines show a Drag response typical of "Indicolite". The gem pictured below (left) shows only a Weak response when floated, and the gem on the right shows only a Strong response. We can speculate that the dark blue color in the gem on the left may be primarily influenced by inter-valence charge transfer between iron ions (Fe2+-Fe3+), while dark blue-green color in the gem on the right may be controlled more by the concentration of iron (Fe2+) ions.
Dark Blue Tourmalines
(.92ct., Weak, SI 69 & .911ct., Strong, SI 218)
Green: Green is the most common color of transparent gem Tourmaline. Color ranges from "grass" green to bluish green to "Emerald" green, and color intensity can range from very light to very dark. Dark green "Verdelite" Tourmaline is colored by dispersed ions of iron (Fe2+ derived from mixing with Schorl) in conjunction with iron-titanium (Fe2+ - Ti4+) charge transfer.
Light Green (4.1ct., Moderate, SI 69) &
"Verdelite" (4.1ct., Drag, SI 278)
Light Green (1.15ct., Moderate, SI 117) &
"Verdelite" (.79ct., Drag, SI 317)
Grayish green and blackish green: Tourmalines of this color are fairly common. Below are 3 examples. The over-dark green hexagon on the left shows a combination of green and gray colors. The color may be influenced by natural radiation. Studies have shown that lab irradiation of some green Tourmalines can produce gray color (Kurt Nassau, 1975). This may involve alteration of the valence state of iron from Fe2+ (green) to Fe3+ (gray), as well as to Fe2+-Ti4+ charge transfer.
The magnetic response of the hexagonal gem (left) is Weak. The blackish green trillion in the center shows a Strong magnetic response. These 2 faceted Tourmalines have magnetic susceptibilities that are similar to lighter green gems. In fact, if the black component could be removed, these Tourmalines might show only light green color.
© Kirk Feral 2013, All Rights Reserved. These materials may be duplicated for educational purposes only. No part of this website may be duplicated or distributed for profit, for commercial purposes, or for posting to another website without the expressed written consent of the copyright holder.
Blue-green "Indicolite"
(5.25ct., Drag, SI 447)
Chrome Tourmaline (1.58ct., Inert, SI <0) &
Pale Green Tourmaline (1.15ct., Moderate, SI 117)
9.2ct.
Light Green (5.06, Weak, <20 SI) &
"Verdelite" (5.94ct., Drag, SI 360)
When a handheld spectroscope is applied to the gems above, we see no definitive absorption spectra that might help us separate the 2 species of green Tourmaline. Besides a magnetic wand, a Chelsea filter is another definitive tool that can easily help us identify Chrome Tourmalines, as these gems show a red or pink reaction under a Chelsea Filter. A blue laser pointer is another tool we can use. Even though vanadium usually quenches UV fluorescence from chromium in dark green Chrome Tourmalines, red fluorescence is evident under concentrated blue laser light.
One Chrome Tourmaline with an unusual brownish green or olive-green color was also encountered in our study, as pictured below. This diamagnetic gem shows pink fluorescence from chromium under longwave UV light. Vanadium and chromium are confirmed with a spectrometer and Chelsea filter as the causes of green color. The cause of the brownish secondary hue has not been determined, but it is most likely due to trace amounts of iron or perhaps iron-to-titanium charge transfer.
Lighter blue colors and shades colored by iron are also found among Tourmalines. Light-colored gems that are greenish blue and powder-blue can be appropriately referred to simply as blue Tourmalines rather than as "Indicolite". The magnetic susceptibility of these blue Tourmalines corresponds to the intensity of color. The two gem triads pictured below show a progression of increasing color saturation from near-colorless to dark blue. Magnetic susceptibility (SI) of the gems in each triad also progressively increases from left to right.
Much of the dark blue portion on the left side of this bi-color gem is opaque, except at the facet edges where it is thin and transparent. This blue portion shows a Drag response. It also shows color change (dichromatism). When viewed in intensely bright incandescent transmitted light, the opaque dark blue color becomes transparent lavender (reddish light + blue body color = lavender). Opaque black Schorl has also been reported to appear lavender when cut in thin section. Surprisingly, the completely colorless portion on the right side of this gem retains enough iron (Fe3+) to show weak to moderate magnetic attraction, indicating that the iron present in that portion of the gem is cryptic.
Blue Becomes Lavender in
Transmitted Incandescent Light
Moderate Drag Drag
Weak Strong Drag
Indigo Blue "Indicolite"
(.41ct., Drag, SI 382)
Blackish Green Tourmaline Rough
(17.62ct., Strong, SI 286)
Tourmalines:
The blackish brown Tourmaline round from Sri Lanka pictured below (left) appears opaque and black in reflected light and can easily be mistaken for black Schorl. Transmitted light (back lighting) reveals that this gem is actually transparent and dark brown. The gem shows a Moderate magnetic response, and we can speculate that this may be due to "surplus" iron that is not engaged in iron-titanium charge transfer. Perhaps for the same reason, the translucent brown rough Tourmaline on the right shows a Weak magnetic response. The magnetism could also be partly due to manganese (Mn2+).
Opaque Black in Reflected Light &
Transparent Brown in Transmitted Light
The 2 brown gems shown below are both strongly magnetic. The oval gem on the left also has yellow and green color, while the pear-shaped gem on the right is yellowish brown. The yellow color in these gems is likely due to manganese, whose presence is suggested by spectrometer analysis. Manganese as Mn2+ can impart strong magnetic susceptibility, as we will show in several other color varieties of Tourmaline.
(1.32ct., Moderate, SI 78)
Yellowish Greenish Brown
(6.65ct., Strong, SI 265)
Yellowish Brown
(2.41ct., Strong, SI 221)
Orange is often a component color of brown Tourmalines. On rare occasions, orange is the dominant color as depicted by the 2 brownish orange gems below. Like brown Tourmaline colored by iron and Fe2+-Ti4+ charge transfer, these brownish orange Tourmalines show no attraction to a magnet.
Orange Tourmaline
(1.1ct., Diamagnetic, SI < 0)
The pinkish-brownish-orange Tourmaline pear pictured below (left) also shows no magnetic attraction, as we would expect. The square Tourmaline on the right has similar color, but it shows a Drag response. In fact, it is the most magnetic transparent Tourmaline tested in this study. How is this possible? The causes of color for this gem cannot be definitively determined with a spectrometer, but we can guess that manganese is primarily responsible for the exceptionally strong magnetism. Manganese can contribute to the magnetic susceptibility of Tourmalines of any color without necessarily contributing to color.
Pinkish Brownish Orange
(2.1ct., Drags, SI 547)
2.9ct.
Pinkish Brownish Orange
(.62ct., Diamagnetic, SI <0)
Orange Tourmaline
(0.75ct., Diamagnetic, SI < 0)
(7.75ct., Weak)
Bluish green color is sometimes seen in light green Tourmalines of the Elbaite species. In these instances, color appears to be due to a mixture of iron Fe2+ ions (blue) and iron to titanium (Fe2+-Ti4+) charge transfer (green). Below are 2 examples. The trillion on the left is only moderately magnetic, while the shield on the right shows a Drag response. The difference in magnetic susceptibilities of these 2 gems of similar color is due to a higher concentration of iron in the gem on the right.
Bluish Green
(3.65ct. Moderate, SI 65)
Bluish Green
(1.6ct, Drags, SI 382)
Blackish Green Tourmaline
(2.69 ct., Strong, SI 130)
In green Tourmalines colored by iron, there is a correlation between color intensity and magnetic susceptibility, just as there is with blue Tourmalines colored by iron. Light green gems show much weaker magnetic responses than dark green "Verdelite" gems (see 3 examples below). We can assume that light green gems are Weak to Moderate due to relatively low iron content, while dark green gems show either a Strong response or a Drag response due to high iron content.
"Verdelite" Tourmaline
(19.46ct., Strong, SI 295)
Note: Besides helping us to identify or separate gems, a magnetic wand provides a means to quickly and efficiently flag gems with unexpected magnetic properties, such as the blue Tourmaline ovals above. We can isolate these gems as candidates for further analysis in order to uncover what is different about them in terms of causes of color.
Black Tourmaline Rough Crystals 17mm
The rough Tourmaline crystal shown above (right) appears opaque black in reflected light and could easily be mistaken for Schorl. But in transmitted light the rough appears translucent blackish-green. It is the most magnetic of the three Tourmalines above, but still not strong enough to show a Drag response characteristic of dark green "Verdelite" gems and opaque black Schorl. The primary species composition of this crystal could be Elbaite, Dravite or Uvite.
Brown Tourmaline Rough Crystal
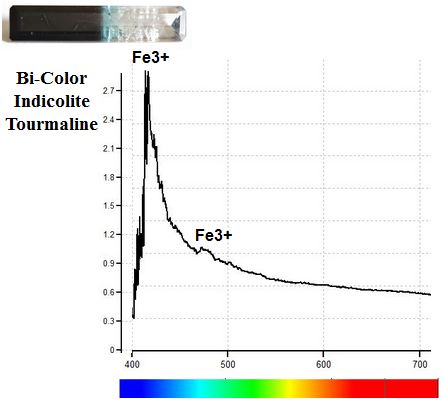
Cryptic Iron: Trivalent Iron (Fe3+) is a weak chromophore, and lower concentrations of Fe3+ may not produce color in some gems. These Fe3+ ions can appear invisible or cryptic, as we see in the colorless end of this Indicolite Tourmaline. Such cryptic iron also does not absorb enough light to show detectable absorption lines under a hand-held spectroscope, but the iron can be detected with more sensitive instruments such as a spectrometer or a magnetic wand. The spectrometer graph below clearly shows the presence of Fe3+ in the completely colorless end of the Tourmaline.
Iron (Fe3+) in a Completely Colorless Section of Tourmaline
Increasing Concentration of Iron in 3 Dark Blue Tourmalines
Spectrometer analysis comparing the two oval gems above to an "Indicolite" cushion supports our hypothesis. The graph below shows that the gem above right has a higher iron content than the gem above left, and that both have a lower concentration of iron than a true "Indicolite" cushion, which shows a drag response.
When we compare the relative magnetic susceptibilities of green Tourmalines to blue Tourmalines, we find that light green gems are on average less magnetic than light blue gems. Dark green "Verdelite" and dark blue "Indicolite" are equally magnetic.
Brownish Green Chrome Tourmaline
Daylight and UV Fluorescence
(0.17ct, Inert, SI <0)
Chrome Tourmaline belongs to the Dravite species, and is a much rarer type of green Tourmaline than green Elbaite. These Dravites typically show dark green color. Most Chrome Tourmalines are colored primarily by vanadium ions (V3+) along with a much smaller percentage of chromium. Vanadium is derived from mixing with the idiochromatic Vanadium-Dravite species, while chromium ions (Cr3+) are derived from mixing with the idiochromatic Chromium-Dravite species.
Because of the low total concentration of both vanadium and chromium in transparent Chrome Tourmaline, the primary species composition of most of these gems is Dravite (or in rare cases Uvite) rather than Vanadium-Dravite or Chromium-Dravite. Chrome Tourmalines are mostly iron-free and diamagnetic, but some are faintly attracted to a magnet, presumably due to the combined magnetic susceptibility of tiny amounts of iron, vanadium and chromium.
Chrome Tourmaline (1.02ct., Inert, SI <0)
Daylight and UV Fluorescence
"Verdelite" Tourmaline
(1.02ct., Drag, SI 295)
Chrome Tourmaline (1.57ct, Inert, SI <0)
Lower concentrations of vanadium and chromium can result in atypical light green colors in Chrome Tourmaline. The two unusual green Tourmalines shown below were sold as Chrome Tourmalines, although neither one shows typical dark chrome-green color. The reaction under a Chelsea filter is faint pink, and there is no LWUV fluorescence, confirming that the light green color is due primarily to low concentrations of vanadium rather than chromium.
Although both of these gems are colored primarily by small amounts of vanadium, Chelsea filter and fluorescent reactions suggests that the light green gem on the right has a somewhat higher ratio of vanadium to chromium than the moderate green gem on the left. Neither gem contains any magnetically detectable iron, and the vanadium/chromium content is also magnetically undetectable. These light green Chrome Tourmalines are both diamagnetic, and both fluoresce a weak yellow color under short wave UV light.
Chrome Tourmaline (14.10ct, Inert, SI >0)
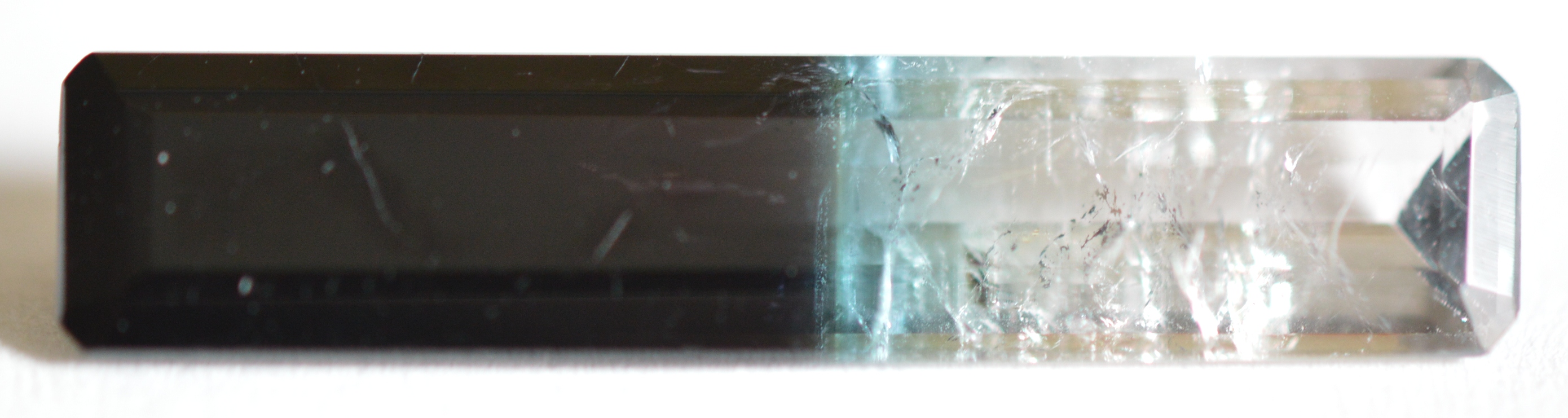
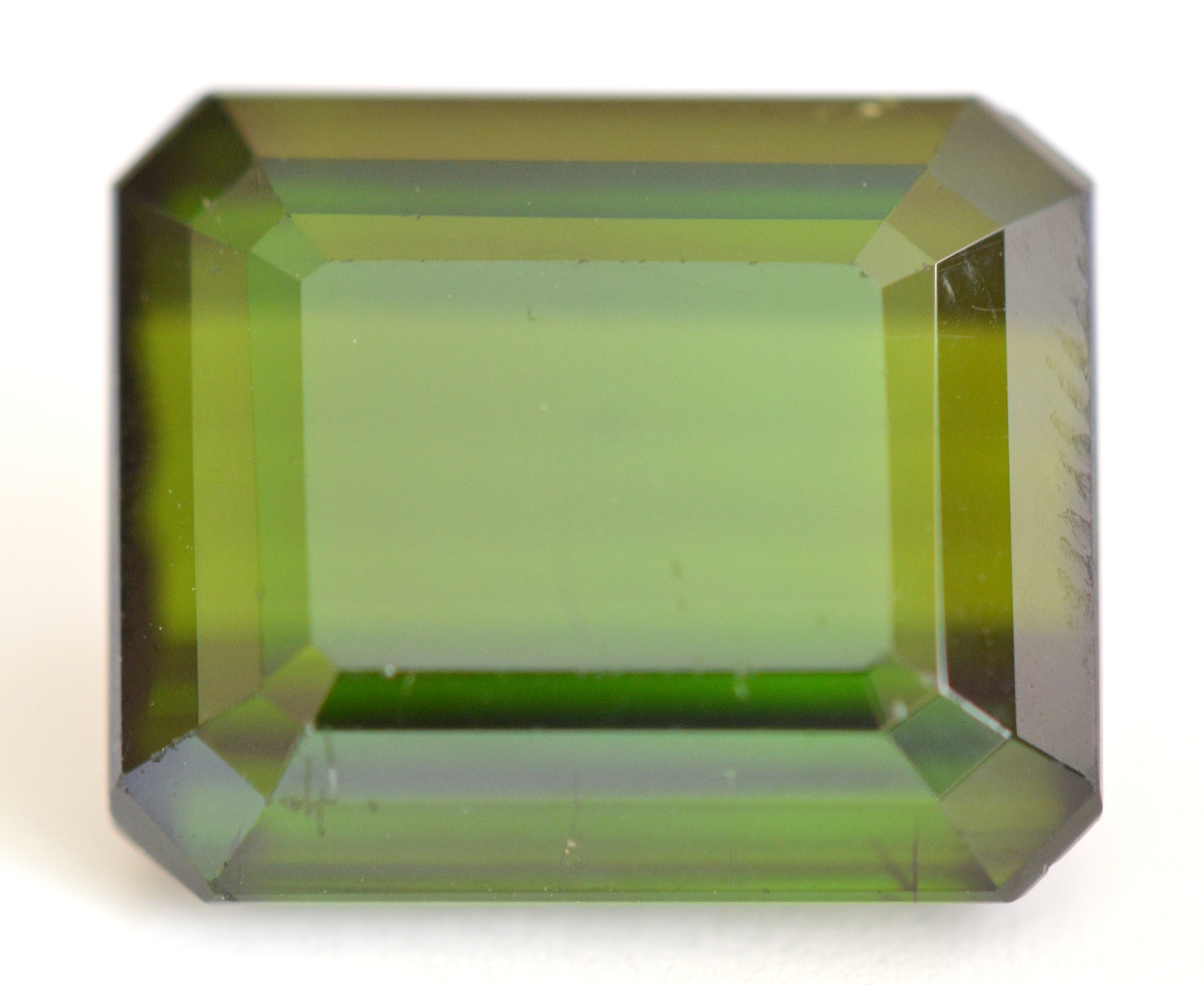
The 3.23ct bi-color Indicolite Tourmaline bar pictured below is quite interesting, so let's take a close look at its identifying characteristics. The body color changes from opaque dark blue on the left side to colorless on the right side of the bar. The refractive index of this gem starts high at 1.63-1.655 on the dark blue end and decreases to the typical Elbaite range of 1.62-1.64 on the colorless end. These unusual changes within a single gem are due to variations in iron content. Lower concentrations of iron (blue color) result in lower densities along the length of the gem, accompanied by lower refractive indices.
Progressive changes in magnetism can also be measured within this bi-color Tourmaline. Measurements taken from left to right at 8 different points along the length of the 24mm bar show a ten-fold decrease in magnetic susceptibility from SI 781 at the dark blue end to just SI 78 at the colorless end due to progressively lower iron content. It is probable that the dark blue portion with susceptibilities above 500 is composed primarily of Schorl (a separate species) rather than "Indicolite" (Elbaite species).
Bi-color Indicolite Tourmaline
Bi-color Indicolite Tourmaline
Even though vanadium is the dominant chromophore, chromium within all Chrome Tourmalines we have tested is detectable as red color under a Chelsea filter. Vanadium apparently has no inhibiting effect on Chelsea filter reactions. By testing the dark green Tourmaline pictured below using a Chelsea filter, UV flashlight and spectrometer, we have determined that this gem is a typical Chrome Tourmaline colored primarily by vanadium. No chromium absorption peak at 680nm is visible with a spectrometer.
Vanadium Quenches Fluorescence: Unlike chromium, vanadium V3+ does not cause red fluorescence under longwave UV light in gemstones of any kind. Instead, vanadium V3+ quenches fluorescence, just as iron quenches fluorescence in other types of gems. For some reason, this important quenching phenomenon is rarely recognized or discussed in gemology.
The gem below clearly demonstrates this phenomenon. This Chrome Tourmaline shows no chromium fluorescence under longwave UV light, but it does show a strong red reaction under the Chelsea filter. This indicates that chromium is indeed present. But any UV fluorescence from chromium is quenched by vanadium. We know the quenching of fluorescence is not due to iron, the universally recognized quencher, as the gem is diamagnetic and contains only a trace amount of iron.
Chrome Tourmaline
Colored Primarily by Vanadium
Vanadium-dravite is an idiochromatic species of Tourmaline that is colored by a high concentration of vanadium. Unlike Chrome Tourmaline of the Dravite species, Vanadium-Dravite is colored only by vanadium, with little or no chromium (verifiable by spectrometer). The gem does not appear red under a Chelsea filter, and does not fluoresce even under blue laser light.
Although crystals of this mineral are rare and generally not of gem quality, we were fortunate to identify an extremely rare example of a faceted Vanadium-Dravite. This gem was bluish green in color, and unlike Chrome Tourmaline of the Dravite species, it showed strong magnetic attraction. We know the magnetic susceptibility must be due to vanadium, as there is no detectable chromium or iron. This is the only example we have found of any gemstone of any kind in which the transition metal vanadium is not only magnetically detectable, but also causes strong magnetic attraction.
Vanadium-dravite Tourmaline Species
(2.53ct., Strong, SI 213)
Pure Blue "Indicolite"
(3.53ct., Drag, SI 356)
Color Change Chrome Tourmaline
Daylight & Incandescent Light
(1.63ct., Inert, SI < 0)
The Chrome Tourmaline shown below has a rich green color typical of this color variety of Dravite, and it is diamagnetic. It not only glows red under a Chelsea filter, it also fluoresces red under longwave UV light due to chromium. Most Chrome Tourmalines do not fluoresce under UV light due to quenching by vanadium. But when a Tourmaline does fluoresce like the one below, it may be an indication that the gem has a higher concentration of chromium relative to vanadium than most Chrome Tourmalines. Chrome Tourmaline is the only variety of Tourmaline that can occasionally fluoresce red under longwave UV light. All Chrome Tourmaline we tested fluoresce red under concentrated blue laser light due to chromium.
Although these dark green Tourmalines are referred to as Chrome Tourmalines, chemical analyses have shown that vanadium is usually the primary cause of color. Vanadium Tourmaline would be a more accurate designation. Even though most dark green Chrome Tourmalines sold in the marketplace are actually Vanadium Tourmalines, the trade name Chrome Tourmaline has been established, and that's not likely to change.
In some instances, the ratio of chromium to vanadium is higher than usual, and in rare instances, Chrome Tourmalines can actually contain a higher concentration of chromium than vanadium. The latter are accurately referred to as Chrome Tourmalines.
In rare instances, Chrome Tourmalines from Umba Valley, Tanzania can show color change from green to reddish brown when incandescent light is applied, and these are referred to as Color Change Tourmalines. Below is a Color Change Chrome Tourmaline in daylight and incandescent light. Spectrometer analysis indicates this gem contains more vanadium than chromium. Although this gem appears bright red under a Chelsea filter due to a small amount of chromium, no red fluorescence from chromium is possible under longwave UV light because any potential fluorescence is completely extinguished by vanadium.
Chrome Tourmaline, 5.35ct
(Inert, SI < 0)
Blue tourmaline gems typically belong to the Elbaite species, but a rare iteration of dark blue Tourmaline that belongs to the Dravite species has recently been found in Afghanistan. A sample of heavily included but transparent blue Dravite (pictured below) reportedly from Badakhshan, Afghanistan that this author tested showed only weak magnetic susceptibility.
Blue Dravite from Afghanistan
1.9 ct, Weak, SI 55
Blackish Green Tourmaline
(5.36 ct., Weak, SI 74)
Magnetism in Gemstones
An Effective Tool and Method for Gem Identification
© Kirk Feral